The following situation will probably be familiar to you: not projectable, sudden bottlenecks, demand of working capacity in business-critical areas or short to medium term peaks in work load of high performers in departments dealing with processes of regulatory relevance or procedures which are time-sensitive for your business operations.
You wish to adequately and flexibly act and find a solution which fulfils all your requirements to manage the shortness of resources: exactly covering your demand, highly qualitative and experienced, short-term availability. At the same time, you do not wish to increase the headcount of your organisation because the critical situation will be time-limited and the accumulating workload is not plan- or foreseeable.
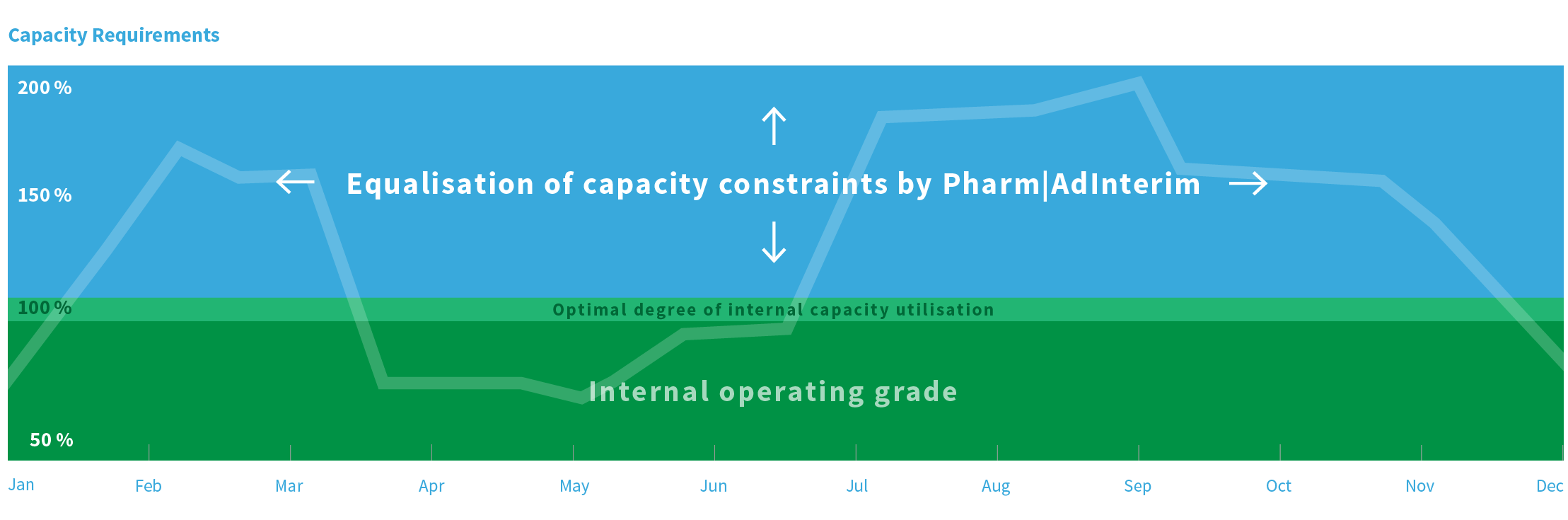
Pharm|AdInterim offers an efficient as well as economic solution: to complement your internal capabilities by external experts – tailored for the function, project or the consultancy mandate for exactly the required period of time.
Pharm|AdInterim provides a network of personalities with outstanding experience in several areas and managing functions (global and national) in the pharmaceutical industry – Medical Affairs, Marketing & Sales as well as Pharmacovigilance, Regulatory Affairs, and GCP-Quality Assurance.
Flexibility in Terms of What, Where and When
“Pharm|AdInterim provides a just as efficient as economic solution to you by expanding your internal know-how with external experts: custom-made for the role, the project, or the consulting assignment – exactly for the required period of time.”
Services Ad Interim
Client Benefit Through…
Succesfully Managed Interim Projects…
Locational Advantage
Wiesbaden is located in the center of the Rhine-Main area. Accessibility of clients’ locations in Europe within half a day by plane, train or car. Frankfurt International Airport just half an hour ride away. Offices located in the city center on the so called “Rue”, parking space available. In this way highly flexible services can be provided on-site at the client’s location, in the Wiesbaden offices or in a combination of both.

